Scott n. Gettinger1, Matthew D. hellmann2, Frances A. shepherd3, Scott Antonia4, Julie Brahmer5, Laura Q. chow6, Jonathan Goldman7, Rosalyn Juergens8, Hossein Borghaei9, Neal E. ready10, David E. gerber11, Faith,,bristol‐myers squibb, Nathan12, Yun Shen12, Christopher Harbison12, Naiyer Rizvi13
1Medical Oncology, Yale
Cancer Center, 2Memorial Sloan Kettering
Cance, 3Princess Margaret Cancer
Centr, 4H. Lee Moffitt Cancer
Center & Research Institute, 5The Sidney Kimmel Comprehensive
Cancer Center at Johns Hopkins, 6University of
Washington, 7University of
California, Los Angeles, 8Juravinski Cancer Centre
at McMaster University, 9Fox Chase Cancer
Center, 10Duke University Medical
Center, 11UT Southwestern Medical
Center, 12Bristol-Myers
Squibb, 13Memorial Sloan Kettering
Cancer Center
Objective:Nivo, a fully human IgG4 PD-1 immune checkpoint inhibitor antibody, has demonstrated durable responses and tolerability in heavily pretreated patients (pts) with advanced NSCLC. This phase I study evaluated the efficacy and safety of nivo monotherapy in pts with chemotherapy naïve advanced NSCLC.
Method: Pts (N=52) with squamous (SQ) or non-SQ advanced NSCLC received nivo 3mg/kg IV Q2W until progression/unacceptable toxicity. Post-progression treatment was allowed per protocol. Response (RECIST v1.1) was evaluated overall, by histology, and by tumor PD-L1 expression (< or ≥ 5% tumor cells expressing PD-L1).
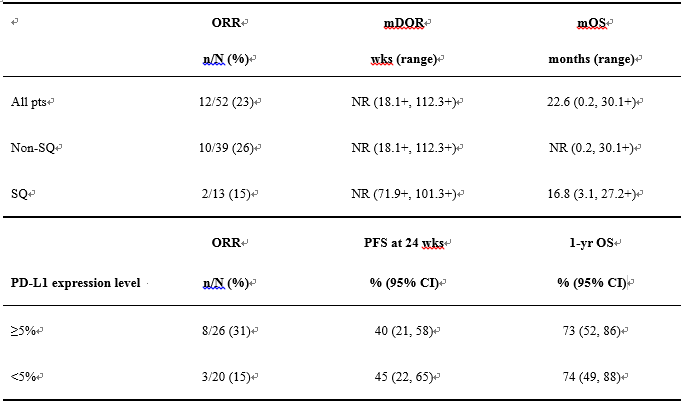
Result: Efficacy by histology and PD-L1 expression are shown (Table). Overall, 9/12 (75%) responders responded by 1st scan (wk 11), and 8 (67%) had ongoing responses at last tumor assessment (median follow-up, 62.2 wks). Four pts had ongoing CRs of 41.4+, 97.6+, 101.3 and 112.3+ wks. Non-conventional immune-related responses were seen in 3 pts, with 35%, 43%, and 46% max reduction in target lesions, respectively, and simultaneous appearance of new lesions (not reported as responders). Similar OS and PFS rates were observed in pts with < or ≥5% PD-L1 expression, although ORR was higher in pts with ≥5% PD-L1 expression. 10 pts (19%) had experienced grade 3–4 treatment-related AEs: rash (n=2), increased amylase/lipase, increased AST/ALT, hyperglycemia, cardiac failure, lung infection, pneumonitis, dehydration/diarrhea, and hyponatremia (n=1 each).[Table].
Conclusion: 1st-line nivo demonstrated durable responses,
encouraging survival and a tolerable safety profile in pts with advanced NSCLC.
Although ORR was higher in pts with ≥5% PD-L1 expression, survival rates were
encouraging across PD-L1 expression levels. Efficacy for additional PD-L1
expression levels will be presented.
Key
Words: nivolumab NSCLC PD-L1

Copyright © 1998 - 2026 Chinese Society of Clinical Oncology(CSCO). All Rights Reserved
Contact Us
EMAIL:office@csco.org.cn
international@csco.org.cn
Phone:86(10)67726451 (Beijing)
86(25)84547290 (Nanjing)