Abstract: At the 2022 Annual Meeting of the American Society of Gynecological Oncology (SGO) held from March 18 to 21, the HLX10-011-CC201 study, led by Professor Lingying Wu from the Gynecology Department of National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Science and Peking union Medical College, was selected as an oral presentation. Professor Jusheng An from the Gynecology Department of National Cancer Center/ National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Science and Peking union Medical College presented the results online [1]. This study evaluated the efficacy and safety of serplulimab combined with nab-paclitaxel in patients with advanced cervical cancer who had failed first-line standard chemotherapy, and yielded very encouraging results. Oncology News invited Professor Lingying Wu to interpret the clinical significances of relevant results and envision the development prospects and trends of immunotherapy in the treatment of advanced cervical cancer from the perspective of investigators.


Cervical cancer is the most common cancer of female reproductive system worldwide [2]. The continuous development of clinical studies and approval of novel drugs have brought new hopes to cervical cancer patients. However, the treatment options are still limited currently, especially for recurrent or metastatic cervical cancer patients who have poor prognosis. The results of the HLX10-011-CC201 study presented at this SGO Conference are expected to bring new hopes for them.
Safe and Effective: Serplulimab in Combination with Nab-paclitaxel Showed Encouraging Efficacy and a Manageable Safety Profile
The HLX10-011-CC201 study is a single-arm, multicenter, Phase II study evaluating the efficacy and safety of serplulimab plus nab-paclitaxel in patients with advanced cervical cancer who have failed first-line standard chemotherapy. The study enrolled histologically or cytologically confirmed patients with advanced cervical cancer who progressed after first-line chemotherapy or were intolerant to first-line therapy, with PD-L1 positive (CPS≥1) and ECOG PS 0 or 1. After enrollment, patients received serplulimab (4.5 mg/kg) and nab-paclitaxel (260 mg/m2) every 3 weeks by intravenous infusion. The primary endpoints were objective response rate (ORR) assessed by the Independent Radiological Review Committee (IRRC) based on RECIST V1.1 criteria and safety. The secondary endpoints included ORR assessed by investigators, progression-free survival (PFS), overall survival (OS), and duration of response (DOR). By December 31, 2021, a total of 21 patients were enrolled, with a median follow-up duration of 14.6 months. ORR assessed by IRRC was 57.1%, including 3 complete response (14.3%) and 9 partial response (42.9%).
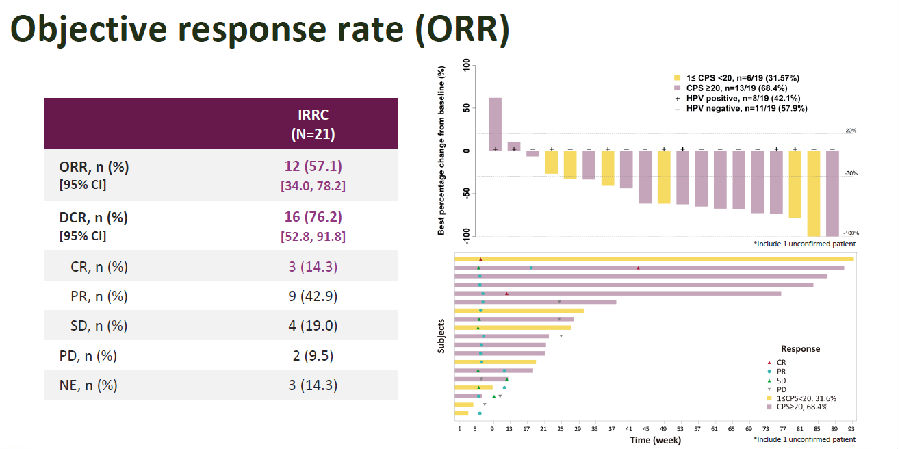 Figure 1. ORR Assessed by IRRC
Figure 1. ORR Assessed by IRRC
As for the secondary endpoints, disease control rate (DCR) was 76.2%; median DOR was not reached, 12-month DOR rate was 70.0%; median PFS was 5.7 months, and 6- and 12-month PFS rates were both 48.2%; median OS was 15.5 months, and 6- and 12-month OS rates were 76.2% and 71.1%, respectively.
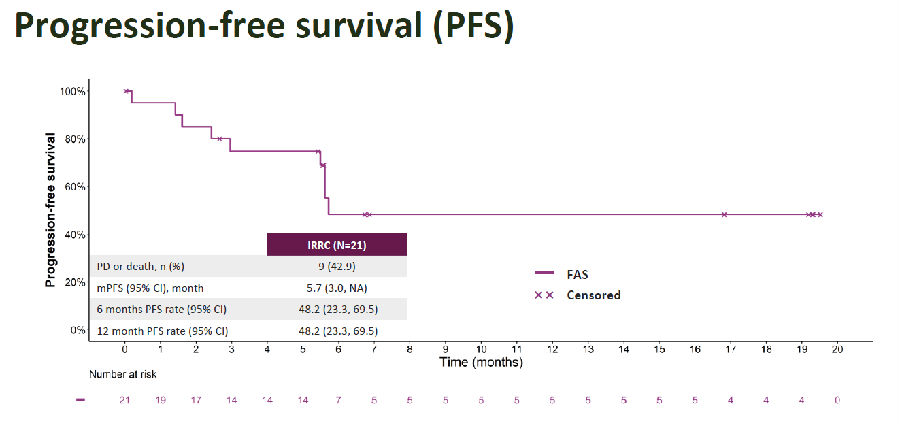 Figure 2. Secondary Endpoint PFS
Figure 2. Secondary Endpoint PFS
 Figure 3. Secondary Endpoint OS
Figure 3. Secondary Endpoint OS
In addition, the safety of the combined regimen was manageable. Data showed that the adverse events were mainly caused by chemotherapy, no grade 4-5 immune-related adverse event (irAE) was observed, and the incidence of grade 1-3 irAE was 9.5%. No patient discontinued serplulimab due to treatment emergent adverse event (TEAE). In general, serplulimab combined with nab-paclitaxel showed promising efficacy and good safety in patients with advanced cervical cancer who had failed first-line standard chemotherapy.
Outstanding Data: Combination of Serplulimab andChemotherapy Had Synergistic Anti-tumor Effect
Comparing to previous studies of immunotherapy orchemotherapy as second-line monotherapy for the treatment of relapsed ormetastatic cervical cancers, the data in this study of serplulimab combinedwith nab-paclitaxel was excellent. To be more specific, in KEYNOTE-158[3]study, PD-L1 positive advanced cervical cancer treated with pembrolizumab onlyreached an ORR of 14.3%, median PFS of 2.1 months, and median OS of 11 months; thesecond-line nab-paclitaxel monotherapy had an ORR of 28.6%, median PFS of 5.0months and median OS of 9.4 months [4]. However, in HLX10-011-CC201study, we observed that the ORR of serplulimab combined with nab-paclitaxel wasas high as 57.1%, the median PFS was 5.7 months, and the 6-and 12-month PFSrates were both 48.2%, reflecting the tailing effect of immunotherapy. Evenmore noteworthy was the 15.5-months median OS, which showed the superiority ofthe combination regimen comparing to those reported in previous studies. Inaddition, the data in this study also reflected the synergistic anti-tumoreffect of the combination of serplulimab and nab-paclitaxel, which bringssurvival benefits and provides a safe and effective new treatment option forpatients with advanced cervical cancer. Serplulimab is the first PD-1 inhibitorwhich obtained OS data of chemo-immunotherapy combinations in Chinesepopulation. The study has further verified the efficacy of PD-1 inhibitor incombination with chemotherapy as second-line treatment for cervical cancerpatients, fully demonstrating the excellent achievements of Chinese investigatorsand innovative drugs.
Positive Significance: Serplulimab Combined with Nab-Paclitaxel is A Good Strategy as Second-Line Treatment for Advanced Cervical Cancer
Firstly, the median OS of this combination was 15.5 months, which is encouraging. Secondly, this study not only demonstrated the synergistic anti-tumor effect of the chemo-immunotherapy combination therapy, but also further verified the excellent anti-tumor efficacy and manageable safety of serplulimab, which can provide evidences for future clinical studies and applications. Thirdly, the combination of immunotherapy and anti-angiogenesis therapy has achieved initial success for relapsed and metastatic cervical cancer patients who failed the first-line chemotherapy and other combination regimens of immunotherapy are still being actively explored. The combination of serplulimab and chemotherapy can provide a potentially better treatment option as second-line treatment for advanced cervical cancer patients.
Great Prospect: First-Line Immunotherapy is highly Anticipated in Cervical Cancer
The benefit of immune-monotherapy is limited for the treatment of advanced cervical cancer. Therefore, combination therapies have become a hot exploring topic and the future trend. First-line immunotherapy is also the focus in the field of cervical cancer treatment. KEYNOTE-826 study [5] confirmed that pembrolizumab combined with paclitaxel + cisplatin/carboplatin ± bevacizumab showed better OS and PFS than those of cisplatin/carboplatin ± bevacizumab as first-line treatment for advanced cervical cancer patients. The PFS in Pembrolizumab arm significantly increased, no matter in patients with CPS≥1, CPS≥10 or the overall population: median PFS were 10.4 months vs. 8.2 months (HR=0.62), 10.4 months vs. 8.1 months (HR=0.58) and 10.4 months vs. 8.2 months (HR=0.65), respectively; The OS in pembrolizumab arm also significantly improved compared to placebo arm in three populations: median OS was not reached vs. 16.3 months (HR=0.64), not reached vs. 16.4 months (HR=0.61), and 24.4 months vs 16.5 months (HR=0.67), respectively. Moreover, the ORR of pembrolizumab arm was higher than the placebo arm: with 68.1% vs 50.2% (CPS≥1), 69.6% vs 49.1% (CPS≥10), and 65.9% vs 50.8% (overall population), respectively. The study suggested that the combination strategy of immunotherapy has a significant benefit for first-line patients with PD-L1 positive advanced cervical cancer. Based on the favorable data from the KEYNOTE-826 study, the US Food and Drug Administration (FDA) approved this regimen on October 13, 2021 as the first-line treatment for patients with persistent, relapsed, or metastatic cervical cancer with PD-L1 expression[6]; the latest version of the NCCN Guidelines for Cervical Cancer in 2022 also included the combined immunotherapy as a recommended first-line treatment for advanced cervical cancer[7]. The studies of immunotherapy in other tumors also showed that the earlier application of immunotherapy had better effects. These strong evidences laid a solid foundation for immunotherapy as a first-line treatment for cervical cancer. It is believed that immunotherapy in combination with other therapies will benefit more cervical cancer patients in the future.

References
AN Jusheng, et al. Efficacy and safety ofserplulimab (an anti-PD-1 antibody) combined with nab-paclitaxel in patientswith advanced cervical cancer who have progressive disease or intolerabletoxicity after firstline standard chemotherapy.2022 SGO.
SUNG H, FERLAY J, SIEGEL R L, et al. Globalcancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwidefor 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021.
Colombo N, Dubot C, Lorusso D, et al.Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N EnglJ Med. 2021;385(20):1856-1867.
Alberts DS, Blessing JA, Landrum LM, et al. PhaseII trial of nab-paclitaxel in the treatment of recurrent or persistent advancedcervix cancer: A gynecologic oncology group study. Gynecol Oncol.2012;127(3):451-455.
Colombo N et al. Pembrolizumab plus chemotherapyversus placebo plus chemotherapy for persistent, recurrent, or metastaticcervical cancer: randomized, double-blind, phase 3 KEYNOTE-826 study. ESMOCongress 2021, Abstract LBA2.
FDA approves pembrolizumab combination for thefirst-line treatment of cervical cancer. [EB/OL].https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-combination-first-line-treatment-cervical-cancer,2021-10-13
NCCN Clinical Practice Guidelines for Cervical Cancer(V1 version 2022)

Copyright © 1998 - 2026 Chinese Society of Clinical Oncology(CSCO). All Rights Reserved
Contact Us
EMAIL:office@csco.org.cn
international@csco.org.cn
Phone:86(10)67726451 (Beijing)
86(25)84547290 (Nanjing)